Biomedical Chromatography-09 November 2018 款冬酮
時(shí)間:2020-09-03 瀏覽次數: 分享到:
Pharmacokinetics and tissue distribution study of bisabolangelone from Angelicae Pubescentis Radix in rat using LC–MS/MS
期刊名:Biomedical Chromatography
文獻編號:DOI 10.1002/bmc.4433
文獻地址:https://onlinelibrary.wiley.com/doi/abs/10.1002/bmc.4433
發(fā)表日期:09 November 2018
 Abstract
A sensitive and accurate LC–MS/MS method was established for quantifying bisabolangelone in rat plasma and tissues. Bisabolangelone was isolated and purified from Angelicae Pubescentis Radix. The pharmacokinetic and tissue distribution of bisabolangelone after administration to rat was performed by LC–MS/MS. Separation was carried out on a C8 (4.6 × 100 mm, 1.8 μm) column. The MS/MS transitions of bisabolangelone and tussilagone (internal standard) were set at m/z 249.1 → 109.1 and m/z 391.4 → 217.4, respectively. The lower limit of quantification in plasma and other tissues ranged from 1 to 4 ng/mL. The biosamples were prepared using protein precipitation method with acetonitrile. The recovery was >92%. The results showed that values of maximum concentrations and area under the curve depended linearly on the studied doses (2.5, 5 and 7.5 mg/kg body weight). The other ingredients in Angelicae Pubescentis Radix extract possibly reduce the absorption of bisabolangelone in rat. Tissue distribution revealed that bisabolangelone was widely distributed in vivo. The highest and lowest concentrations of bisabolangelone were found in the stomach and in the brain, respectively. It was concluded that the newly established HPLC–MS/MS method was suitable to describe the pharmacokinetic characteristics of bisabolangelone in rat after administration
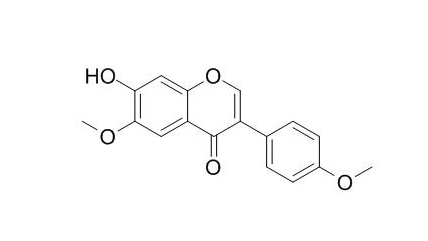
Tussilagone [internal standard (IS), purity > 98.9%] was purchased from Chengdu Desite Biological Technology Co., Ltd (Chengdu, China)
Abstract
A sensitive and accurate LC–MS/MS method was established for quantifying bisabolangelone in rat plasma and tissues. Bisabolangelone was isolated and purified from Angelicae Pubescentis Radix. The pharmacokinetic and tissue distribution of bisabolangelone after administration to rat was performed by LC–MS/MS. Separation was carried out on a C8 (4.6 × 100 mm, 1.8 μm) column. The MS/MS transitions of bisabolangelone and tussilagone (internal standard) were set at m/z 249.1 → 109.1 and m/z 391.4 → 217.4, respectively. The lower limit of quantification in plasma and other tissues ranged from 1 to 4 ng/mL. The biosamples were prepared using protein precipitation method with acetonitrile. The recovery was >92%. The results showed that values of maximum concentrations and area under the curve depended linearly on the studied doses (2.5, 5 and 7.5 mg/kg body weight). The other ingredients in Angelicae Pubescentis Radix extract possibly reduce the absorption of bisabolangelone in rat. Tissue distribution revealed that bisabolangelone was widely distributed in vivo. The highest and lowest concentrations of bisabolangelone were found in the stomach and in the brain, respectively. It was concluded that the newly established HPLC–MS/MS method was suitable to describe the pharmacokinetic characteristics of bisabolangelone in rat after administration
Tussilagone [internal standard (IS), purity > 98.9%] was purchased from Chengdu Desite Biological Technology Co., Ltd (Chengdu, China)